| Author | Message | ||
Chio U Kim Username: Chioukim Registered: 04-2003 |
Dear ILCian, Just tell me your problems about Chemistry. I am right here to discuss with you your problems. | ||
Chio U Kim Username: Chioukim Registered: 04-2003 |
Is this a redox reaction? Na2CO3 + 2HCl ® 2NaCl + CO2 + H2O | ||
Chio U Kim Username: Chioukim Registered: 04-2003 |
It is not. Since there is no change in oxidation number | ||
Chio U Kim Username: Chioukim Registered: 04-2003 |
Try this S4 on-line exercise | ||
Chio U Kim Username: Chioukim Registered: 04-2003 |
Try this also if you are S5 students ® S5 on-line exercise | ||
20013001 Username: 20013001 Registered: 04-2003 |
many questions here since I can stay annoynmus. 1. During electrolysis, why H20 is splitted into H+ and OH- instead of H2+ and O2-? So when does H20 change to H. OH and when does h2o changes to H2, 0? 2. How do you tell which pole is +ve or -ve during eletroylsis? 3. What is polar/ non-polar solvent? that's all for now... more coming. | ||
Chio U Kim Username: cuk Registered: 04-2003 |
1.H2+ does not exist because H has only one electron. When H2O splits into H+ and OH-, one of the O-H covalent bond of H2O molecule is broken and the two shared electrons go to the oxygen leading to the formation of H+ and OH-. 2. The positive electrode (anode) of an electrolytic cell always attracts the negative ions (anions). The negative electrode (cathode) of an electrolytic cell always attracts the positive ions (cations) 3. The most common polar solvent is water. Non-polar solvents include tetrachloromethane(CCl4), hexane(C6H14)...etc.. Polar solvent can dissolve polar substances (e.g. HCl); non-polar solvent can dissolve non-polar substances (e.g. I2). The so-called 'like dissolves like' rule. | ||
951129 Username: 951129 Registered: 04-2003 |
Dear Mr Chio or Mr Wong, i would like to ask a question about dynamic equlibrium. Is there any changes on the Kc when the temperature is increased for the endo or exo rx? THX | ||
951129 Username: 951129 Registered: 04-2003 |
Besides, is that the pressure will affect the time to achieve the equlibrium? In the chapter Chemical Kinetic in New way textbk, it told us that pressure is a factor to change the rx rate. But why the table (in modern physical chemistry P.269) said there is only little effect in liquid? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Kc is temperature dependent. If the forward reaction is endothermic, Kc increases as temperature increases. If the forward reaction is exothermic, Kc decreases as temperature increases Rate of Reaction involving gases is affected by pressure. Thus, the time taken to achieve an equilibrium state is affected by pressure. However, the equilibrium constant is NOT pressure-dependent. The effective concentrations (or the densities) of liquids and solids are independent of pressure. So, the rates of reactions involving only liquids or solids are not affected by pressure. For example, CaCO3(s) ® CaO(s) + CO2(g) the forward reaction is not affected by pressure whereas the reversed process is speeded up by an increase in pressure. | ||
��"mo"�J~Michael Username: 981087 Registered: 04-2003 |
May i ask a question? When NaCl is dissolved in water Na+ and Cl- ions are mobile in the aqueous solution. If potassium is dissovled in water, will K+ and O2- ions be formed? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Potassium reacts with water to give potassium hydroxide (K+ and OH-) and hydrogen gas. | ||
981039 Username: 981039 Registered: 04-2003 |
Can alkanol react with sodium or other metals? | ||
�D�@���D�@���D�@���D Username: 981039 Registered: 04-2003 |
to give out salt and water? | ||
Inamoto5 Username: admin Registered: 04-2003 |
Some alkanols (small carbon nos.) can reacts with sodium or some other reactive metals....one typical example is 2CH3CH2OH + 2Na --> 2CH3CH2O-Na+ + H2(g) Usually, we use this method to dispose the useless sodium. | ||
Inamoto5 Username: admin Registered: 04-2003 |
The name of the salt CH3CH2O-Na+ is called Sodium ethoxide.... | ||
��"mo"�J~Michael Username: 981087 Registered: 04-2003 |
�ݿ���...���n�N�� �ګY�Q��potassium oxide �����|�Xwhat ions? �|���|�XO2- | ||
Chio U Kim Username: cuk Registered: 04-2003 |
It gives OH- because O2- is very unstable in water and reacts with water molecules to give hydroxide ions. O2- + H2O ® 2OH- | ||
��"mo"�J~Michael Username: 981087 Registered: 04-2003 |
HCl(g) NH3(g), CO2(g), SO2(g) are they acid? | ||
Inamoto5 Username: admin Registered: 04-2003 |
HCl(g),SO2(g) and CO2(g)...we call these gases...acidic gases. After they have dissolved in water..they becomes hydrochloric, sulphrous and carbonic acid respectively..... NH3(g) of course is not an acidic gas...it can dissolve in water to form ammonia solution... Besides, ammonia can partially ionize to form ammonium and hydroxide ions... | ||
Inamoto5 Username: admin Registered: 04-2003 |
...please pay attention hydrochloric acid is a strong acid but sulphrous acid and carbonic acid are only weak acids because their molecules are only partially ionized in water....^;^ | ||
981907 Username: 981907 Registered: 04-2003 |
How do we know that when Iron reacts with acid or anything, the product is iron (11) or iron(111) ions? | ||
991035 Username: 991035 Registered: 04-2003 |
If some metals and non-metals do not appear in the e.c.s. How can we indicate them?? thx | ||
991035 Username: 991035 Registered: 04-2003 |
What is the difference between the sturcture of metal 2+ and metal3+?? | ||
991035 Username: 991035 Registered: 04-2003 |
In the chemcial cell reaction, Elctrode is Zn and Cu Electrolyte is CuSo4 Why Cu 2+ will accept the 2e- but not So4? The electrochemical serise of So4 tell us that it's the easiest to accept the electron.... | ||
991035 Username: 991035 Registered: 04-2003 |
Will Mr M.C. Wong go to school tomorrow? thx | ||
Inamoto5 Username: admin Registered: 04-2003 |
When iron metal reacts with acid (if reaction can occur), Fe2+(aq) ion will be produced. Fe2+(aq) ion can be oxidized to Fe3+(aq) ion if a strong oxidizing agent is used (e.g. dichromate or permanganate). H+(aq) ion is not strong enough to oxidize the Fe2+ ion. | ||
Inamoto5 Username: admin Registered: 04-2003 |
If you can not find the metal or non-metal ions in the E.C.S of your textbook, it means that you can ignore them....(will not appear in your examination) | ||
Inamoto5 Username: admin Registered: 04-2003 |
I don't understand the question about structure... you are wrong, please pay attention to the half equation of SO42- ion.....S2O82-(aq) + 2e- <=> 2SO42-....S2O82-(aq) accepts electron...not 2SO42- | ||
Inamoto5 Username: admin Registered: 04-2003 |
I hope the S.6 classmates can help to answer question about cert. level...^;^ This can enhance your concept..... Come on..all S.6 Chem students.... | ||
Issa Username: 971047 Registered: 04-2003 |
Coming | ||
Inamoto5 Username: admin Registered: 04-2003 |
Thank You... | ||
971067 Username: 971067 Registered: 04-2003 |
Coming Soon~~ | ||
991160 Username: 991160 Registered: 04-2003 |
�����@d�W�Ǵ��N�Ať�ѬJ�P�Ǿ����O.. �L�קڦa�Ӯa�I�d��ť��..���Y����..~ �n���զӮa�J�����Y�n���n��..~ �ڵo�{�ۤvڻ�Y�|�@�Y��..~ ���Y�S�Ӯa�Z��ut�S���Y�n�h�ɶ�..~ �s�����N�i�H�l�o��~~? | ||
�ڭ}�� Username: 971067 Registered: 04-2003 |
�A�Y���|�ǥ�~?? �̮a�ܰl����N��b�Y�Ӧ���~ ��ꤤ�|�W�Ǵ���D�����J��~ �n���f��������~ �u�n�A���ߪַŪ�Ū~ �ݴN�N���D�[��~ | ||
991160 Username: 991160 Registered: 04-2003 |
�Y��~�ګY���|��~ ���~���Y�Ӯa�N��ut���{..~ ���֤����Jut�S�n...���~? �r~ �I�˧�ӦWga~? �ګü�J�Y..~ �A�I�˥�971067�ﰵ�ڭ}��~? | ||
���� yan..~* Username: 991160 Registered: 04-2003 |
�u�u�u��~~~ �ڪ��D�I���~@@" | ||
�ڭ}�� Username: 971067 Registered: 04-2003 |
�u�n���߷�~~ �@��N�űo��Book 1A + 1B��~~ �����Y�űo�ͭ��Y�̭��n~~ �̭��n�Y���\����~~ ���Ѩ����t�[~~ �@�wڻ�o���[~~ �դU�q�ɶ��h���\��~~ | ||
981036 Username: 981036 Registered: 04-2003 |
�������ȶ��Y�k�H��~ | ||
�ڭ}�� Username: 971067 Registered: 04-2003 |
Dear Mr Kwok(981036), �դUo�YBio�G�פS���N�A�ɶ�~ ���Y�S���ɶ�����H~ �P��{�ɶ�o�Y�O��~ �ˤ��p�q�hD�ɶ��Ůѧa��~ �̫�Ҧ��I�Y�ݩ�A�ۤv�[�Q~ ��D�H��n�h�ɶ���Chem~ �]���\�ڥ��N�����Gpart�����A~ �ҥH���_�����ݥhڻ�G�q�Ҥ�~ �ڦۤv���չL~ ���L�A���եh���\����~ �ݷť��P�N�Ŧ������O�O~?? �ܲM�����dmessage��~ "�������ȶ��Y�k�H��"~ �ȶ��Y�k�H~ ���X��ǥͳ���~ ���Y�I�Ѩȶ��@�w�n�Y�k�H~ ���X��ǥͭ��|��~ �@�ӤH���hڻ�hD�ѭ��h�ǦhD��~ �S�I�|���է�h�Ǩ��h�O~?? | ||
�ڭ}�� Username: 971067 Registered: 04-2003 |
Sorry~ �Y������~ ���L�A�����եh���\����~ �ݷť��P�N�Ŧ������O�O~?? | ||
991097 Username: 991097 Registered: 04-2003 |
�ڷQ�ݤ@�ӽҥ~�J�D��ar!! �����ڻ�@�Ӧ�����Ǫ��`�ءA�\��咗�ӹ���: �N�@�M�@�ئP�@�M�B��J�L�i�l�Aafter 4 mins�A�M�@�ؼ������A���M�B�N�L����A�ӥD��������(use english)�A���ڳ����Y�n��ar!! Can anybody answer me ar??Thx!!! | ||
Chio U Kim Username: cuk Registered: 04-2003 |
The following explanation is just a guess because I am not sure about the details of the experiment. I think it can be explained in terms of the molecular motion. In solid state (e.g. Ice), the molecular motion is minimized and thus is the resonance between the molecules and the microwave. | ||
Inamoto5 Username: admin Registered: 04-2003 |
First of all, you should know the principle about microwave oven. Under the microwave,the water molecules (in liquid state) will become resonance (rotation).(frequency of microwave = rotational frequency of the molecule). Energy is released as heat. I guess that in solid state, the open cage structure of ice, the rotational frequency of the molecule will shift to other values. Moreover, in this structure, the gaint ice molecule will not easy to rotate (the molecular motion is minimized) | ||
991137 Username: 991137 Registered: 04-2003 |
Dear sir~~~ I'd like to ask a question about the electrolysis of water~~~ we add the sulphric acid to the water to make an ionic condition in order to increase the conductivity~~~~ but how could this break the covalent bond of water so that the H+ and OH- will be attracted to the cathode and anode individually? THX for answering my question~~~ | ||
Chio U Kim Username: cuk Registered: 04-2003 |
In pure water, water molecules are constantly colliding with one another. If the molecules collide with sufficient kinetic energy, the O-H covalent bonds will be broken resulting in the formation of hydrogen ions and hydroxide ions according to the following equation. H2O + H2O ® H3O+ + OH- However, the concentrations of hydrogen ion and hydroxide ion are extremely low (1x10-7M at 298K). So the electrolysis of pure water is not apparent. The addition of sulphuric acid makes it easier to observe the evolution of gases during electrolysis. | ||
991011 Username: 991011 Registered: 04-2003 |
what is universal indicator ar? under what condition will it change its colour? | ||
�ڭ}�� Username: 971067 Registered: 04-2003 |
An universal indicator is prepared by mixing a number of indicators. As different indicators show colour chane at different pH range, the mixture shows different colours from pH 1 to 14. | ||
���� yan..~* Username: 991160 Registered: 04-2003 |
�ڭ}��~ �ڳ������ѫY�n~ �ڳ����n�֥i�Hڻ�{����.. ���Y���D�Yڻ����...~ | ||
Samson Username: 981125 Registered: 04-2003 |
�����ݧڰ� book one�n�� | ||
���� yan..~* Username: 991160 Registered: 04-2003 |
���ګY���ѪGd balance�r..half equation�r.. �`���Y�@�³��[�@�³��Gd�ڴN�����Y����.. �ڤW�Ǵ��Y�����Nť�L��...�n�ᮬ~"~ | ||
Samson Username: 981125 Registered: 04-2003 |
Chem�ݦnŪ�A����Ū=.= �L�p��,�A�u�n���T��M��book one A,B�\ (�p�G�A�^��n����) | ||
���� yan..~* Username: 991160 Registered: 04-2003 |
�rsir�W��j��...�N�߾�ťo_o" (just kidding) �ڭ^���Y�̦n�G��=_= ���Y�u�Yڻ�o��--? �Z��ut���Y�n�h�ɶ�... �ӥB...�\�ҤS���i... | ||
�ڭ}�� Username: 971067 Registered: 04-2003 |
To ���� yan..~*: �p�G�A�^��n~~ ��A�ŮѦ��n�j�u�լ[~~ Chem��D�r������n�`~~ ��A�������Ӻ�靈�l~~ �ڬ۫H�̤j���D���Y���Y�n�M��o�Jo��~~ �W�Ǵ�D���n�l�f�u�Y�����[~~ �p�G�A�u�Y�����s��~~ �ݴN�@�w�n���f���e�GD��~~ ������A�h�դU���s�GD~~ ���Y�u�Y�|�U���U�����[�Q~~ | ||
���� yan..~* Username: 991160 Registered: 04-2003 |
���~ �h�¦U������J������U��~ �p�G�ڭ��n�n�ݰl��d chem.. �Y�}�|�n������A�a�O~? | ||
Samson Username: 981125 Registered: 04-2003 |
�ڦa�S���|���A�Y��� ������ڦa���A�ҿ� ���Chem���n�h���쪺�a�� ���M�ڤ��|��Chem�h�ƪ� ���L�ڹ�o�즳�`�p������ ���|�����Z���ӭ��n �̭��n�Y���ɤ��� �����summer���}ݯ��T��M���\�o | ||
���� yan..~* Username: 991160 Registered: 04-2003 |
���~ ���D���D^v^ | ||
991137 Username: 991137 Registered: 04-2003 |
Dear Sir~ i don't really understand when the NACI is being electrolyted with the electrode of mercury, why the NA+1 will be reduced and form an alloy with the mercury but not when the electrode is graphite? THX for answering my question. | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Mercury, being a liquid metal, tends to dissolve other metals such as sodium to give alloys of mercury (amalgams). Graphite, being a solid non-metal, cannot form alloys with metals easily. In other words, mercury can be considered as a good solvent for dissolving metals(just like water is a good solvent for dissolving salts). By forming an alloy with mercury, the reactive sodium can be stabilized. This is why sodium ions are discharged rather than hydrogen ions at the mercury cathode. | ||
991035 Username: 991035 Registered: 04-2003 |
dear everybody I wanna ask a question. when acidified potassium permanganate solution do redox reaction with Fe2(SO4)3 The Fe2+ will do oxidation to lose electrons to become a Fe3+. But when Fe2(SO4)2 solution be a electrolyte and do the redox reaction with zine anode and Fe cathode, the Fe ion will do the reduction. WHY? is it Fe ion can do both job oxidation and reduction? THANK all | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Fe3+ acts as an oxidizing agent when it reacts with a reducing agent such as zinc. Fe2+ acts as a reducing agent when it reacts with an oxidizing agent such as MnO4-. Remember : Fe3+ cannot be oxidized even in the presence of a strong oxidizing agent such as MnO4-. because the higher oxidation number of Fe is +3. Question : Do you think Fe2+ can act as an oxidizing agent in the presence of a reducing agent such as Zn ? | ||
991035 Username: 991035 Registered: 04-2003 |
If it can happen, (-) At anode Zn(s)------> Zn2+ + 2e- (+) At cathode Fe2+ + 2e- -----> Fe(aq) But can Zn be powerful enough to reduce the Fe2+ ? and do Fe2+ need a strong reducing agent to discharge? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Zn is above Fe in the electrochemical series. It is a displacement reaction! Zn, being a more reactive metal (a stronger reducing agent), can displace the less reactive Fe ( a weaker reducing agent) from a solution containing its ions (Fe2+). | ||
991035 Username: 991035 Registered: 04-2003 |
Then my anwer above is correct, right?? but I forget the displacement reaction suddenyl............ Would you make more questions for us to prastice, please??? thabk you so much! | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Have you tried this link? http://www.ilc.edu.hk/SubjectWeb/ChemWeb/S4%20Ex/index.htm | ||
991035 Username: 991035 Registered: 04-2003 |
Yes I have | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Well done | ||
�� Username: 991934 Registered: 04-2003 |
��sir,��sir,�ڷQ���p�G��electrolysis�ɡA���I�ѭ��i�H�Ψ�ӹq���H�H�H �P�I�i���i�H���U����@�������J������....Thz~~~ | ||
991035 Username: 991035 Registered: 04-2003 |
Mr wong, Would you please tell us that the mean of the UT in 4B, and also how many people get 40 above?? In the whole form, getting 40 above is good or only average?? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Mean of 2nd UT of 4A : 30.8 '���I�ѭ��i�H�Ψ�ӹq���H�H�H' Please clarify your question ! | ||
Inamoto5 Username: admin Registered: 04-2003 |
Mean of 2nd UT of 4B is 27.0...not so bad! Only 2 classmates got 40 above....they are Yip Ka Wa and Lee Kam Wa....2 WaWa!! ^3^ | ||
�� Username: 991934 Registered: 04-2003 |
���n�N��r��sir,��ڻ����making scheme������ӧڭ��Y���ӹq���A�ӫY�����N�[ammeter & rhestat,�ڲשۤv��d����...... thz~~~~~ | ||
991035 Username: 991035 Registered: 04-2003 |
���pݯd ���Q�פU la �n�e�r! | ||
991035 Username: 991035 Registered: 04-2003 |
Mr C.M.Wong Can you post the answers of class practice in chemist 200?? So that we can check the answers at home. Thank you! | ||
991035 Username: 991035 Registered: 04-2003 |
Dear all I wanna ask a question. when we make a cake, we add the NaHCO3 and soilid acid in it. ionic equation is: NaHCO3(s) + H+ (aq) ------> H2O(l)+CO2(g)+Na+(aq) if the ionic equation is right, where will the Na+(aq) go when we after finish making the cake? Thank all | ||
Chio U Kim Username: cuk Registered: 04-2003 |
If the solid acid is tartaric acid, sodium ions exist as solid sodium tartrate in the cake. Note that the anion (tartrate ion) of the tartaric acid is not shown in the ionic equation! | ||
991035 Username: 991035 Registered: 04-2003 |
I find this question from a book. I don't know the meaning of that it asks: Give ne use of sodium hydrogencarbonate in first aid process? Mr chio would you please answer my question~ thx | ||
991035 Username: 991035 Registered: 04-2003 |
Excuse me, I wanna sk one more question. When I write the reaction of H2SO4(L) and show out its dehydrating action. Should we only write this? e.g. C6H12O6(S)------------>6C (S)+6H2O(L) or write other more complaicated equation? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
If conc. acid gets onto your skin, you should first wash the affected area with plenty of water and then with a little sodium hydrogencarbonate solution. It is because NaHCO3 can remove the acid by the reaction : H+(aq) + NaHCO3(aq) ® Na+(aq) + H2O(l) + CO2(g) No need to write a more complicated equation for the dehydration of glucose. | ||
991035 Username: 991035 Registered: 04-2003 |
�ڷQ�ݤU�O, �����Y mei �� ga? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Fire is a mix of energy (light and heat) and chemicals (e.g. CO2 and H2). | ||
991035 Username: 991035 Registered: 04-2003 |
�� why fire �|�� shape �J? | ||
991137 Username: 991137 Registered: 04-2003 |
��ť���Ӫŭӫת����Nshape ga wor~~~ | ||
991137 Username: 991137 Registered: 04-2003 |
sir~~~ �ڷQ��acid�Pwater or alkali�Pwater react ������Xo���A��~~~ | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Strong acid + water ® complete ionization e.g. HCl(aq) + H2O(l) ® H3O+(aq) + Cl-(aq) In Cert-level Chemistry, H3O+(aq) is represented as H+(aq) Weak acid + water ® incomplete ionization e.g. CH3COOH + H2O(l) ® H3O+(aq) + CH3COO-(aq) Similarily, Strong alkalis undergo complete ionization in water. Weak alkalis undergo incomplete ionization in water. | ||
991147 Username: 991147 Registered: 04-2003 |
how to find the relative atomic mass of metal X? The formula of a metal carbonate is X2CO3. 100cm3 of a solution containing 0.69g of the carbonate requires 50 cm3 of 0.2 M hydrochloric acid for complete reaction. | ||
Chio U Kim Username: cuk Registered: 04-2003 |
According to the balanced equation, X2CO3 + 2HCl ® 2XCl + CO2 + H2O 1 mole of X2CO3 reacts with 2 moles of HCl No. of moles of HCl reacted = M x V = 0.2 x 0.050 = 0.01 mol Therefore, no. of moles of X2CO3 reacted = 0.5 x 0.01 mol = 0.005 mol. no. of moles of X2CO3 = mass/molar mass => 0.69g/(2X + 12 + 3x16)g = 0.005 => X = 39 So the relative atomic mass of X is 39 | ||
991147 Username: 991147 Registered: 04-2003 |
100 cm3 of X2CO3 reacts with 50 cm3 why we don't need to divide the no. of moles of X2CO3 which is 0.005 mole by 2 | ||
991147 Username: 991147 Registered: 04-2003 |
what i mean is 50 cm3 of HCL | ||
991147 Username: 991147 Registered: 04-2003 |
A metal X forms a hydroxide XOH. 1.12g of XOH were dissolved in some distilled water and then made up to 250 cm3 with distilled water. 25.0 cm3 of this solution required 20.0 cm3 of 0.10 M hydrochloric acid for complete neutralization. What is the relative atomic mass of X? solutions: No. of moles of 20cm3 of 0.1M HCL = 0.002 No. of moles of 25cm3 XOH = 1.12/(y+17)*(25/250)=0.012/(y+17) 0.012/(y+17)=0.002 y=39 At this case, why we need to divide 25cm3 XOH by 10 but the above case don't need. when will we need to aware of the concentration of solution | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Since the mass of X2CO3 is fixed (0.69g), the no. of moles of X2CO3 reacted is also fixed. In this case, whether the volume of X2CO3 used is 100 cm3 is not important. The balanced equation tells us that 1 mole of X2CO3 reacts with 2 moles of HCl, but not 100 cm3 of X2CO3 solution reacts with 200 cm3 of HCl. | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Remember : Since 1.12 g of XOH were in 250 cm3 of XOH solution, only 1.12÷10g of XOH were present in 25.0 cm3 of the XOH solution | ||
Chio U Kim Username: cuk Registered: 04-2003 |
20.0 cm3 of 0.10 M hydrochloric acid react with only 25.0 cm3(but not 250 cm3) of XOH solution. | ||
991147 Username: 991147 Registered: 04-2003 |
do we need to memorize the process and definition of the separation of mixtures? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Yes. | ||
991147 Username: 991147 Registered: 04-2003 |
why zinc hydroxide redissolves in excess sodium hydroxide to give a complex solution? will other metal hydroxide redissolves in escess NaOH also? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Zn(OH)2(s) + 2OH-(aq) ® Zn(OH)42-(aq) Lead(II) hydroxide and aluminium hydroxide also redissolve in excess NaOH. Pb(OH)2(s) + 2OH-(aq) ® Pb(OH)42-(aq) Al(OH)3(s) + OH-(aq) ® Al(OH)4-(aq) Remember, complex formation is not required in Cert-level Chemistry Examination. | ||
991137 Username: 991137 Registered: 04-2003 |
sir~ �ڷQ�ݤU�I��aluminium o�Jdensity �ݧC~~~ ���Y�S��strong o�J�H | ||
Chio U Kim Username: cuk Registered: 04-2003 |
The factors affecting the density of a substance are not the same as those affecting its strength. | ||
�� Username: 991934 Registered: 04-2003 |
��sir, �i���i�H��ڻ,�A��"The factors affecting the density of a substance are not the same as those affecting its strength."�ݫY�}�Hmetal�Jbonding�hڻ,�p�G�Y�J��,�ݥi���i�H�ܷ�metal atom �Jsize�V��,�\�Jmetallic bond strenght�N�|�j...... ��no. of outermost electrons�V�h,�\�Jmetallic bond strenght���N�|�j......o����o���[? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Be careful, the strength of a metal is not equivalent to the strength of its metallic bond. The strength of a metal depends on the arrangement of atoms (structure) and the strength of metallic bond plus others. | ||
Chio U Kim Username: cuk Registered: 04-2003 |
The strength of metallic bond depends on the no.of outermost electrons of the atom and the distance between the outermost electrons and the nucleus. | ||
�� Username: 991934 Registered: 04-2003 |
��sir, �ڷQ��Day 6 ��calculation�Y��d��,�Y�}only ��ch13? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Yes, the test on Day 6 (26/09) is for Chapter 13 only. Of course, you should know how to write balanced equations for the chemical reactions involved in the calculation. | ||
991137 Username: 991137 Registered: 04-2003 |
�ڷQ�� (CH3)3CH O�Jstructural formular �I�g�H thx | ||
Chio U Kim Username: cuk Registered: 04-2003 |
(CH3)3CH is the condensed structural formula of C4H10 A more complete structural formula for C4H10 is : 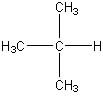 | ||
991054 Username: 991054 Registered: 04-2003 |
�ڷQ�ݤ@�Ӱ��D polythene �Macetate ���Opla��tic���@�� �\�a���Opolymer ��covalent bond ���\�a����������������Ӧnstrong ���ǹq�l���Ӥ��e�����}atom ������u�n�@�g�����A���̪��q�l�A�N�|���}�h��L�a��ΪŮ𤧤��H�H | ||
991073 Username: 991073 Registered: 04-2003 |
A mixture of propanoic acid, methanol and a few drops of conc. sulphuric acid is heated in a beaker of hot water. After 10 mins, the mixture mix with the sodium carbonate solution. Q: State 2 observable changes when the contents of the test tube were added to sodium carbonate solution. A: Effervescence occurs and the presence of smell. I have a few queations: 1) The gas bubbles evolved is CO2?? 2) Why smell is count of observable changes? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Effervescence is due to the formation of carbon dioxide. Heat changes/changes of smell(from odourless) /colour changes/formation of precipitate or gas bubbles are possible observable changes of chemical reactions. | ||
20001186 Username: 20001186 Registered: 04-2003 |
I have 1 question about potassium, sodium and calcium... If one of these metals react with steam, metal oxide + hydrogen or metal hydroxide + hydrogen will be the result? | ||
991147 Username: 991147 Registered: 04-2003 |
why does alloy have a stronger metallic bond but lower melting point? how do we know that an alloy is more corrosion resistant than metal, is it related to metallic bond? what is the relationship between metallic bond and melting point? why do the ends of a nail rust more than the other parts? what do we call the particals in metal? ions or atoms? what is the difference between shelf life and service life? | ||
Inamoto5 Username: admin Registered: 04-2003 |
Inside metal, the particles are ions the ends of a nail rust more...becos of sharp point effect....more ions accumulate at the sharp point shelf life: how long it can last for storage service life: how long it can be used under loading. melting point of metal depends on both bond strength and packing efficiency | ||
991912 Username: 991912 Registered: 04-2003 |
�p�G�nchange CaCO3 to CaSO4 �I�ѭ��i�H�����NCaCO3 react with H2SO4? �N��Y�]��protective layer, �ݦp�G�NCaCO3�[HCl����A�NCaCl�PH2SO4 react ���|�XCaSO4���|�Yprotective layer �������P? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
CaCl2 is soluble in water but CaCO3 is NOT ! On mixing CaCl2(aq) with sulphuric acid, CaSO4 is precipitated and can then be separated from the solution by filtration. | ||
991147 Username: 991147 Registered: 04-2003 |
Q : A mixture contains only copper (II) oxide and anhydrous copper(II) sulphate. Which of the following methods can be used to separate copper(II) oxide from the mixture? 1. Add water to the mixture and them filter 2. Add dilute nitric acid to the mixture and them filter. 3. Add concentrated hydrochloric acid to the mixture and then filter. ANS : (1) only Why (3) cannot be the answer? Will CuO and CuSO4 react will Dilute HNO3 and conc. HCL and why they react? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
The question is about if we can 'separate' the two substances but not about if they react with the acids concerned. | ||
991147 Username: 991147 Registered: 04-2003 |
Why CuSO4 react with conc. HCL? what is the type of this reaction? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
No reaction ! | ||
991147 Username: 991147 Registered: 04-2003 |
CuO is a solid and can react with conc. HCL to form an aqueous solution? but anhydrous copper(II) sulphate remains as a solid, then we can seperate them. but why this is not the case? I would like to ask the second question: is molten state = liquid state? Thanks a lot! | ||
991147 Username: 991147 Registered: 04-2003 |
if the question is 'NAME the apparatus involved?' can I just answer 'Pipette' | ||
991147 Username: 991147 Registered: 04-2003 |
sorry! I would like to ask the question 'Describe how large crystals of ammonium sulphate can be prepared from an aqueous solution of ammonia in a school laboratory.' why the answer to describe the process just state mixing ammonia with sulphric acid in the mole ratio 2:1. Why does not need to state the titration process? when will we need to state the titration process involved to prepare the insoluble salt? | ||
991147 Username: 991147 Registered: 04-2003 |
why the statement ' ethene burns with a sooty flame than hexane ' is correct? C2H4 + 3 O2 gives 2 CO2 + 2 H2O C2H6 + 3.5 O2 gives 2 CO2 + 3 H2O so 1 mole of ethene needs 3 moles of oxygen to have complete combustion, but 1 mole of ethane needs 3.5 moles of oxygen to give complete combustion when can we use the symbol to respresent the spelling the the chemical substance, e.g. NaOH to respresent sodium hydroxide? Do we need to put the state like NaOH (aq) when we use it? | ||
Chio U Kim Username: cuk Registered: 04-2003 |
Testing |